
Oxygen Bohr Model (Diagram, Steps To Draw) Techiescientist
Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr's model calculated the following energies for an electron in the shell, n : E ( n) = − 1 n 2 ⋅ 13.6 eV
Bohr Model Chemical Element Oxygen Atomic Theory PNG, Clipart, Angle, Area, Atom, Atomic Number
The Bohr model represents these energy levels as rings. We can tell that the two electrons in the model above are at the same energy level because they are on the same ring. Limitations. By representing electrons as particles, the Bohr model does not reflect the wave properties of electrons. The electrons appear to exist in specific locations.
Bohr Model Oxygen Chemical Element Atomic Number, PNG, 1000x1000px, Bohr Model, Area, Atom
The Bohr Model was proposed in 1913 by the physicist Niels Bohr and is a description of the structure of atoms in which there is a dense positive core surrounded by orbiting electrons. In this model, the electrons orbit the nucleus in circular orbits, accounting for the series of discrete wavelengths in the H2 emission spectrum.

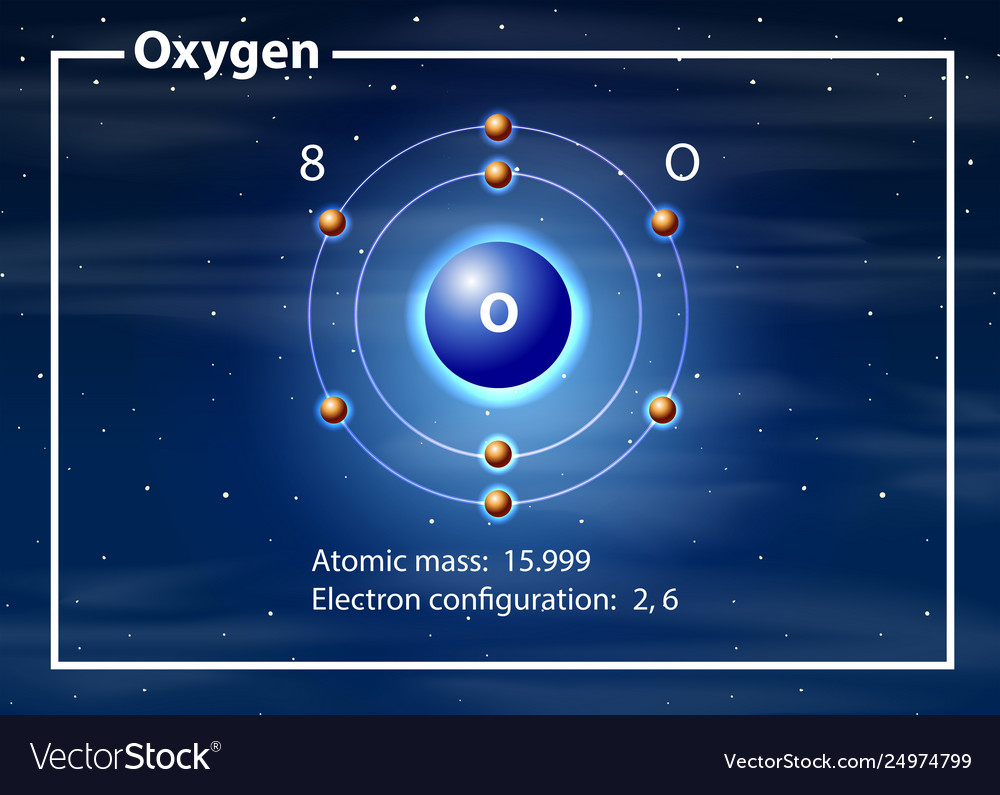
Oxygen, atom model. Chemical element with symbol O and with atomic number 8. Bohr model of
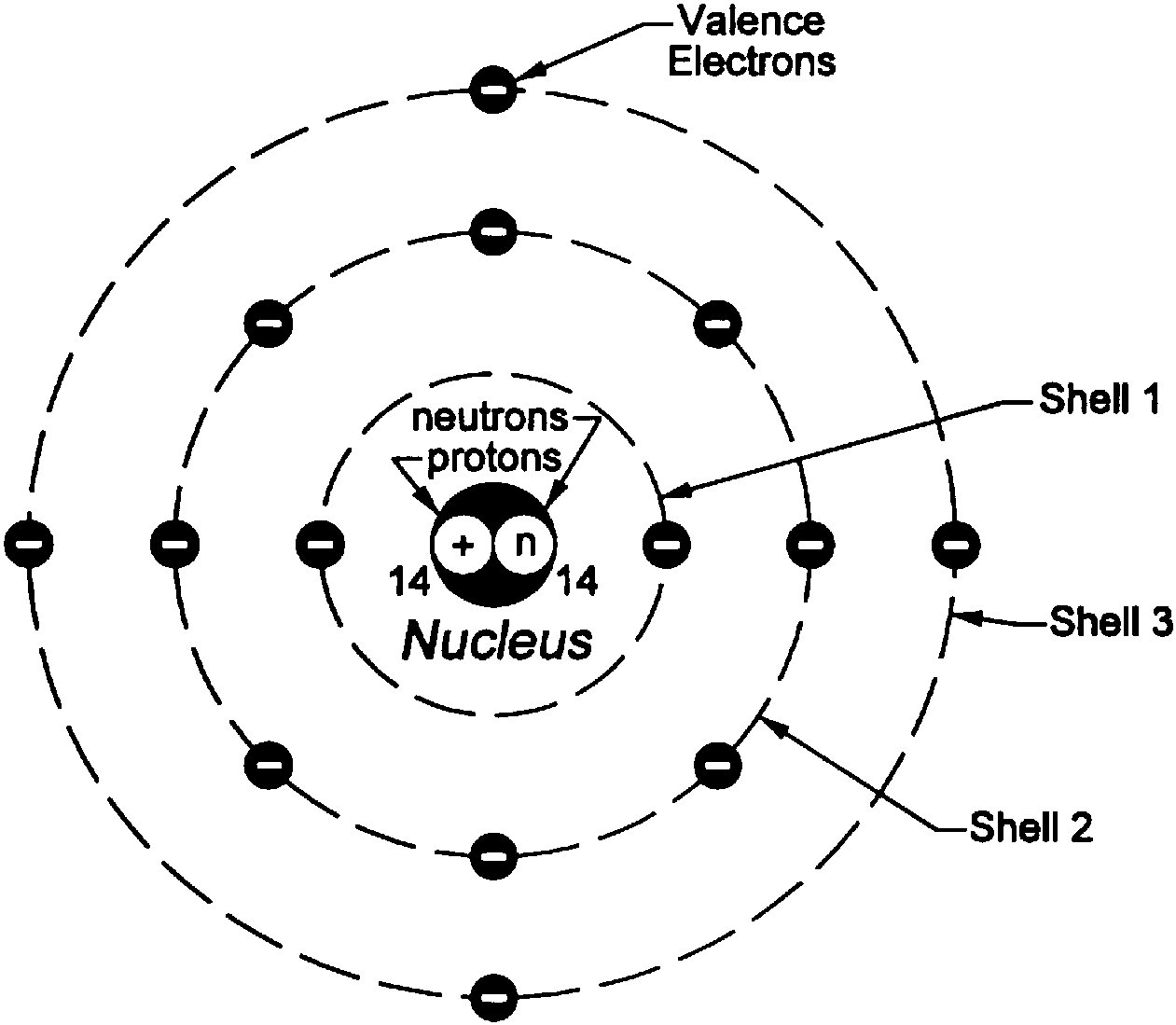
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
Oxygen Electronic Structure lupon.gov.ph
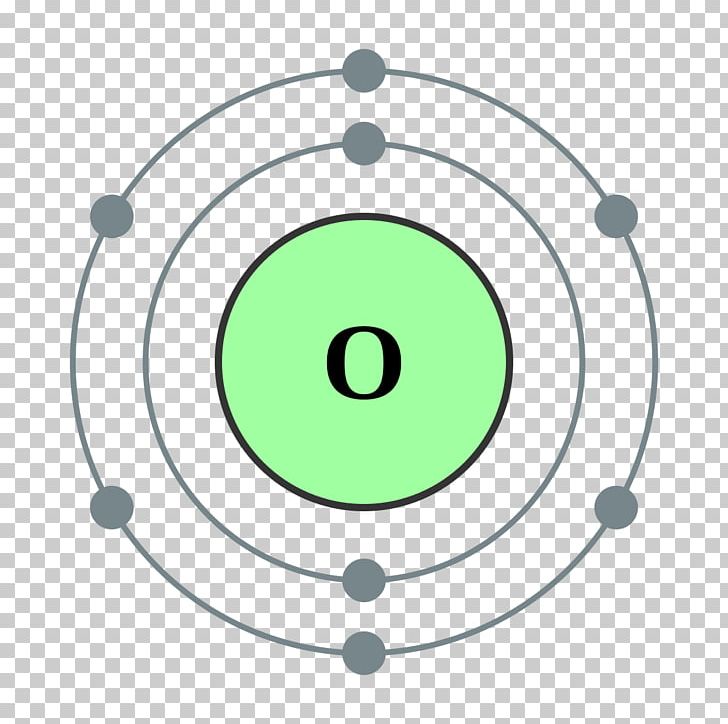
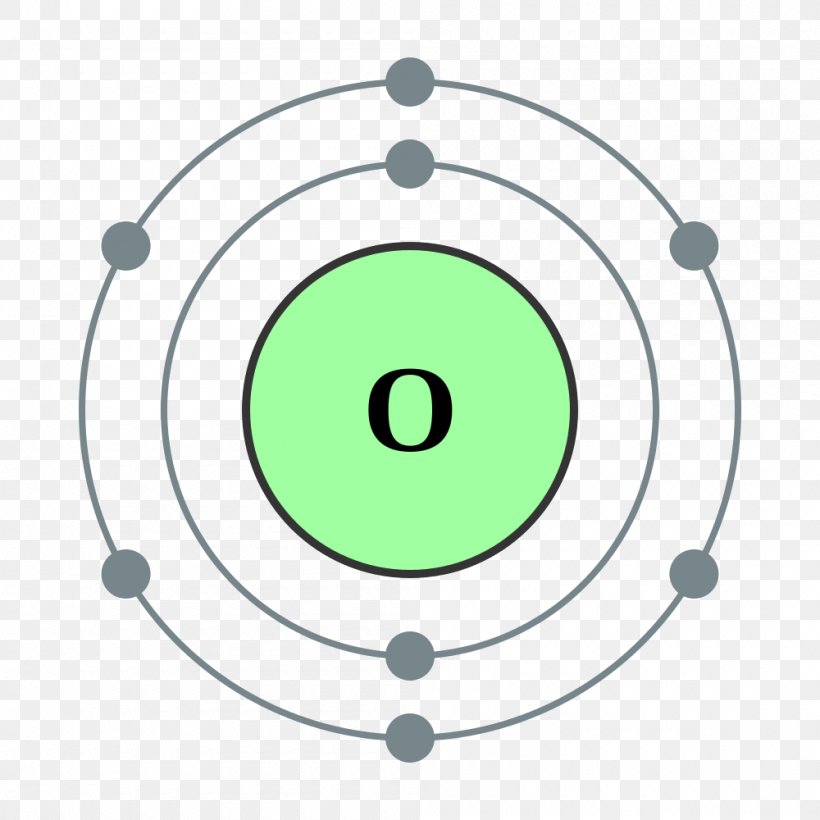
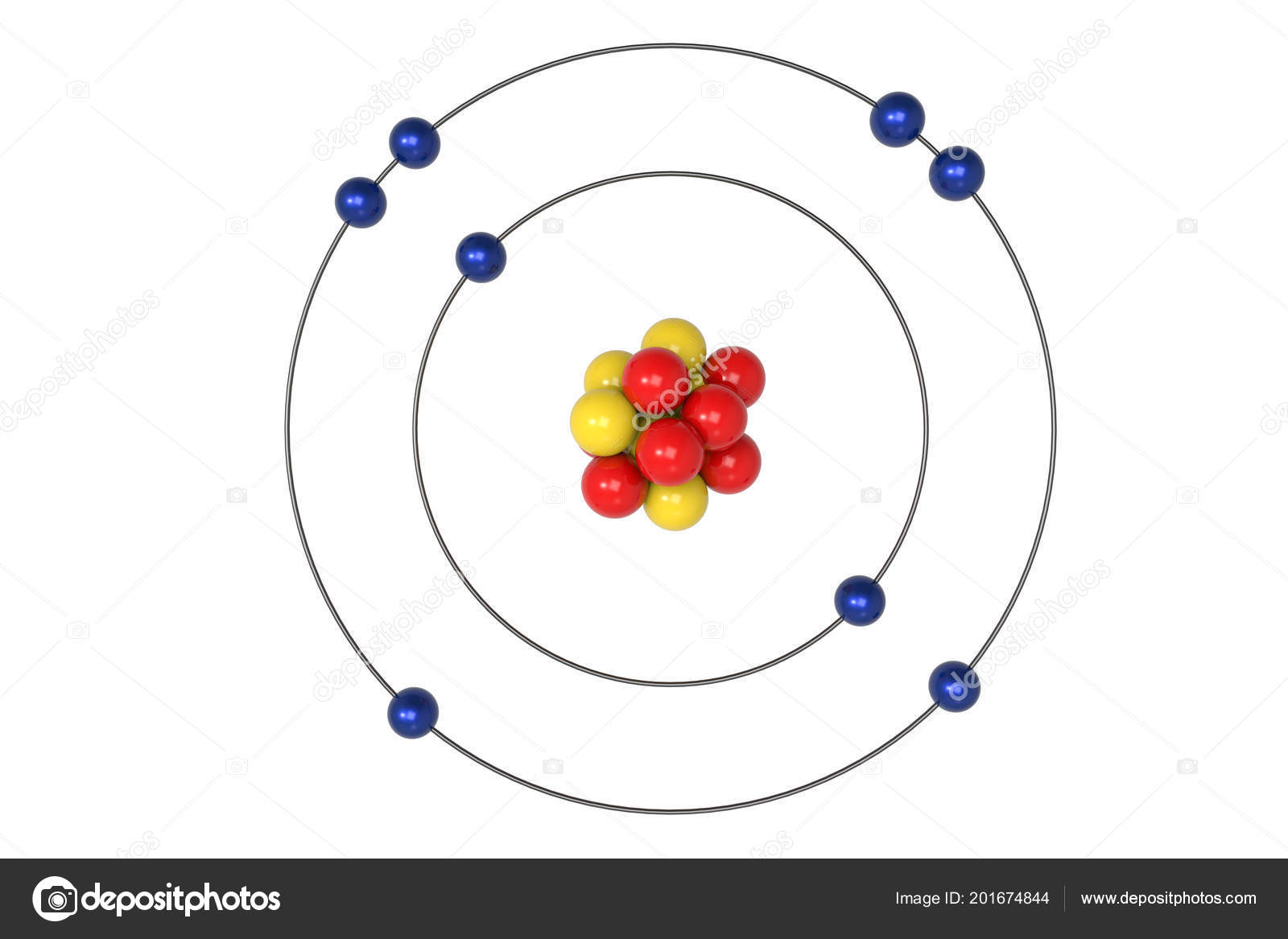
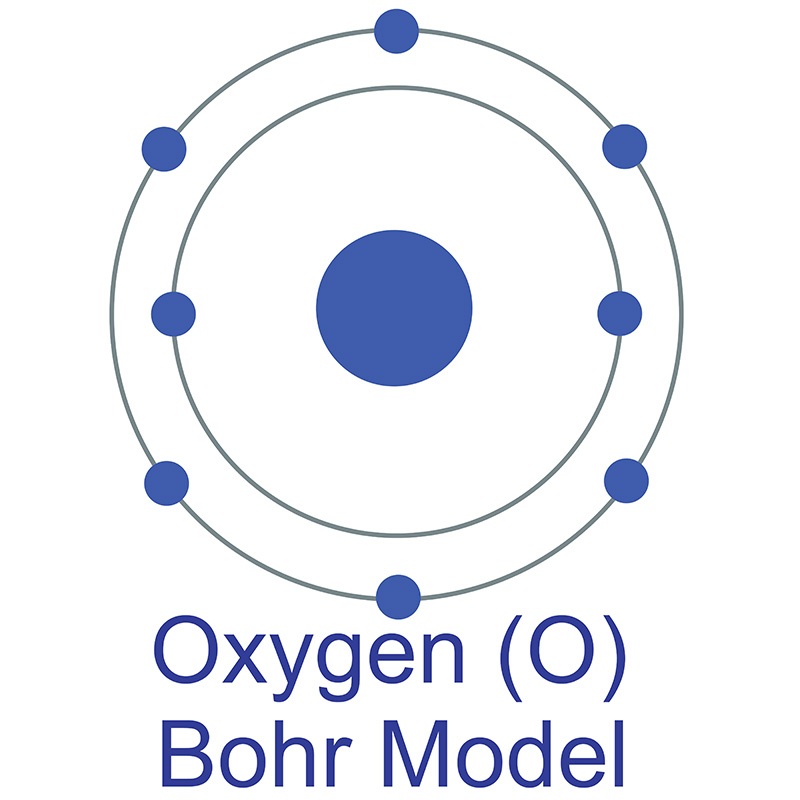
How to draw the Bohr-Rutherford Diagram for Oxygen. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on.

draw a bohr model of an oxygen atom artvansaletoday
Apply: atomic spectra Science > High school chemistry > Atoms, elements, and the periodic table > The Bohr model and atomic spectra Apply: Bohr models Google Classroom A Bohr model of an oxygen atom is shown below. How many valence electrons does the oxygen atom have? Choose 1 answer: A B C Stuck? Review related articles/videos or use a hint.

Oxygen Valence Electrons (O) Oxygen Valency & Electron Configuration
A Model for Atomic Structure: An atom is the smallest particle that makes up matter. It has subatomic particles within it called protons, neutrons, and electrons. The Bohr model, named after scientist Neils Bohr, is a way of illustrating the structure of the atom and the location of its subatomic particles. Specifically, the model shows that.

Oxygen atom Bohr model stock vector. Illustration of white 267662185
Figure 7.3.2 7.3. 2: The emission spectra of sodium and mercury. Sodium and mercury spectra. Many street lights use bulbs that contain sodium or mercury vapor. Due to the very different emission spectra of these elements, they emit light of different colors. The lines in the sodium lamp are broadened by collisions.
Bohr Model Drawing Of Oxygen at GetDrawings Free download
The Bohr Model is known as a planetary model because these orbits look similar to that of planets orbiting the sun. The Bohr Model, Explained There are three main factors that characterize the Bohr model. First, in the Bohr Model, the orbits have a set size and energy.

Diagram representation of the element oxygen Vector Image
Explaining the Bohr Model. In 1924 Louis de Broglie proposed that electrons have a wave nature. As part of that proposal he also described the relationship between the wavelength of the wave aspect and the mass and speed of its particle aspect. The proposal has been experimentally confirmed and is one of the fundamental aspects of Quantum.
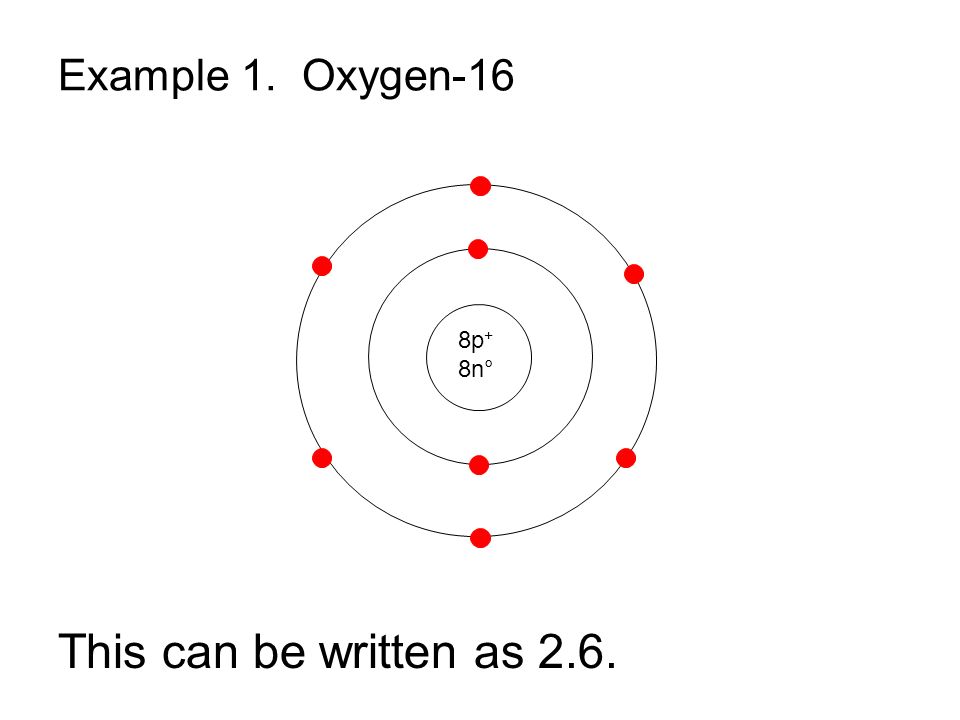
Bohr Model Drawing Of Oxygen at Explore collection of Bohr Model Drawing Of
Category: Science & Tech Key People: Niels Bohr Related Topics: atom On the Web: Space.com - The Bohr model: The famous but flawed depiction of an atom (Jan. 08, 2024) See all related content → Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr.
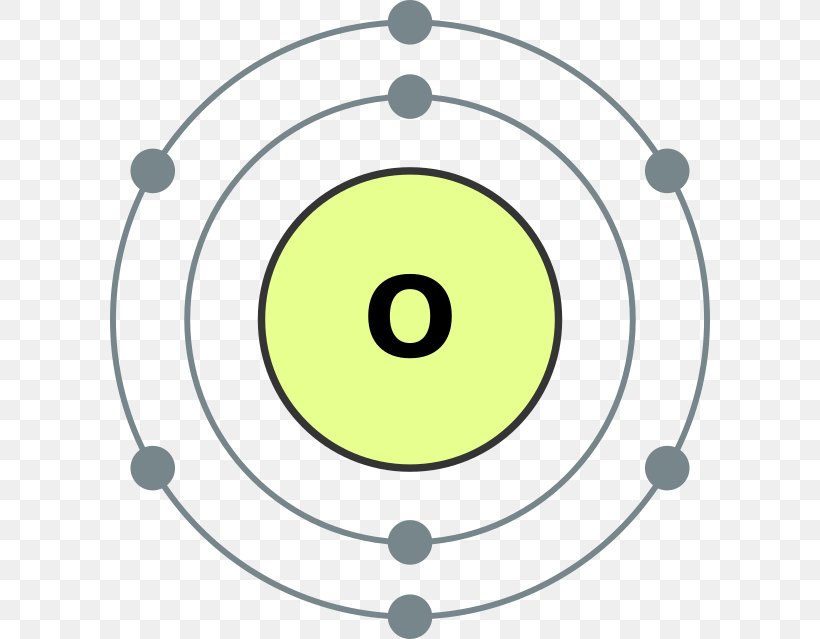
Oxygen Atom Diagram Photos Cantik
The Bohr model of oxygen contains a nucleus having 8 protons and 8 neutrons in the center, and around this nucleus, there are two electron shells containing 8 electrons. Atomic Structure (Bohr Model) for Oxygen (O) Watch on Contents Steps #1 Write protons, neutrons, and electrons of oxygen atom #2 Draw nucleus of oxygen atom
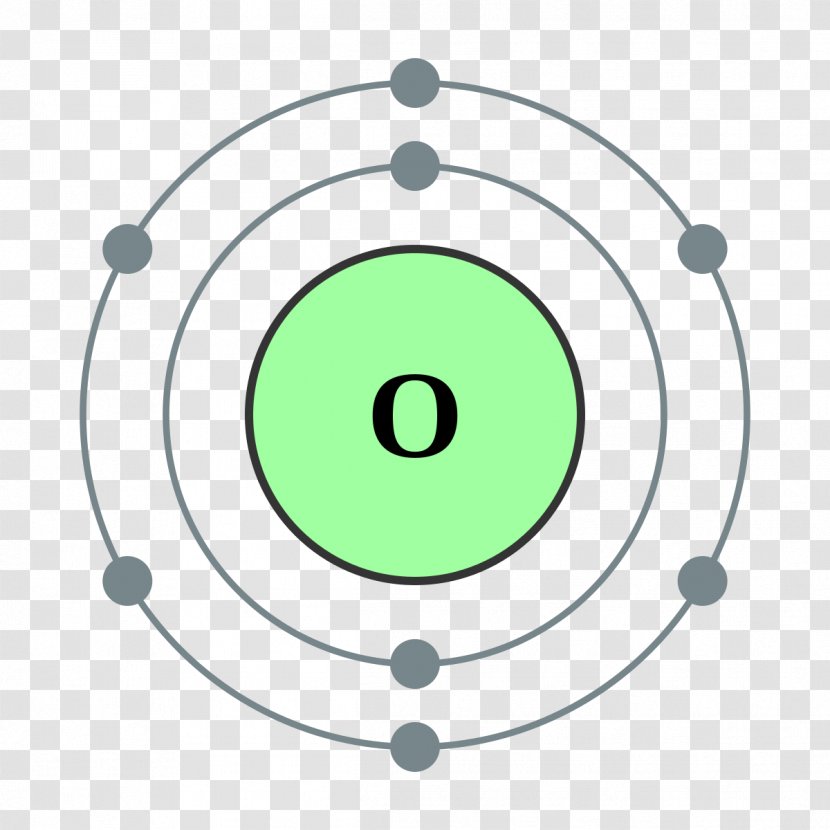
Bohr Model Chemical Element Oxygen Atomic Theory Green Shells Transparent PNG
Atomic Structure (Bohr Model) for Oxygen (O) Wayne Breslyn 725K subscribers Join Subscribe Subscribed 116 Share 22K views 1 year ago In this video we'll look at the atomic structure and.
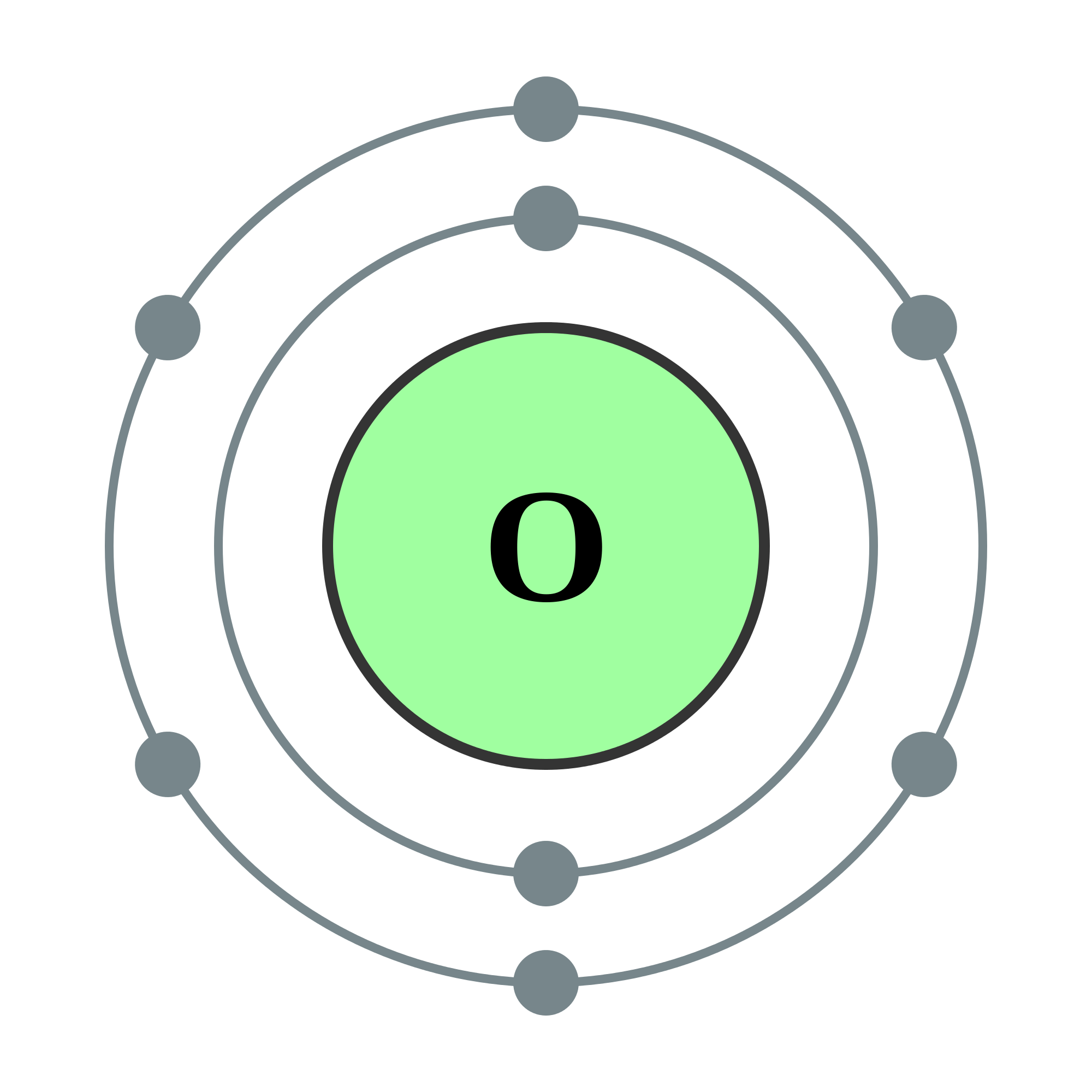
Octet Rule Valence Electrons Of Oxygen Exceptions to the Octet Rule jehomewithanh
One of the weaknesses of Bohr's model was that he could not offer a reason why only certain energy levels or orbits were allowed. Figure 10.4.1 10.4. 1: The energy levels of the electrons can be viewed as rungs on a ladder. Note that the spacing between rungs gets smaller at higher energies (CC BY-NC; Ümit Kaya)
Oxygen atom diagram concept Royalty Free Vector Image
This page titled 5.1: The Bohr Model is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Tom Weideman directly on the LibreTexts platform. Back in the early days of quantum mechanics, Bohr constructed a model of the hydrogen atom that worked surprisingly well considering its simplicity.
Oxygen (O) AMERICAN ELEMENTS
This classical mechanics description of the atom is incomplete, however, since an electron moving in an elliptical orbit would be accelerating (by changing direction) and, according to classical electromagnetism, it should continuously emit electromagnetic radiation.